silicon isotopes|Iba pa : Baguio Silicon (14Si) has 23 known isotopes, with mass numbers ranging from 22 to 44. Si (the most abundant isotope, at 92.23%), Si (4.67%), and Si (3.1%) are stable. The longest-lived radioisotope is Si, which is produced by cosmic ray spallation of argon. Its half-life has been determined to be approximately . Tingnan ang higit pa THE BEST CRAPS TIPS ARE: Go for the pass line bet. Go for the come bet. Go for the don’t pass bet. Go for the don’t come bet. Go for the odds bet. Place the 6 and/or 8. Go with the don’t 6 and don’t 8. Don’t get .
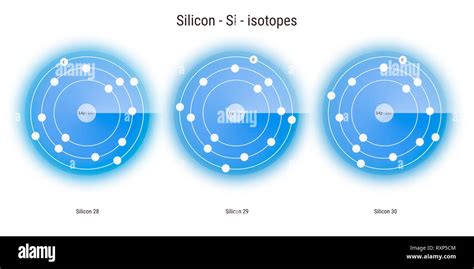
silicon isotopes,Silicon (14Si) has 23 known isotopes, with mass numbers ranging from 22 to 44. Si (the most abundant isotope, at 92.23%), Si (4.67%), and Si (3.1%) are stable. The longest-lived radioisotope is Si, which is produced by cosmic ray spallation of argon. Its half-life has been determined to be approximately . Tingnan ang higit paSilicon-28, the most abundant isotope of silicon, is of particular interest in the construction of quantum computers when highly enriched, as the presence of Si in a sample of silicon contributes toSilicon . Tingnan ang higit paSilicon-29 is of note as the only stable silicon isotope with a nuclear spin (I = 1/2). As such, it can be employed in Tingnan ang higit pa
• Silicon isotopes data from The Berkeley Laboratory Isotopes Project's Tingnan ang higit paSilicon-34 is a radioactive isotope wth a half-life of 2.8 seconds. In addition to the usual N = 20 closed shell, the nucleus also shows a . Tingnan ang higit paIntroduction. Silicon isotope analysis of diatom silica ( δ30 Si diatom) is a rapidly evolving technique for tracking changes in silicon uptake by diatoms within both marine and .Learn about the three stable isotopes of silicon (28, 29, and 30) and how they vary in natural environments. Explore the methods and applications of silicon isotope analysis in .
Learn about the 23 known isotopes of silicon, including the three stable ones and the radioactive ones with various half-lives. Find data on atomic mass, spin, parent nuclide, .A silicon atom has fourteen electrons. In the ground state, they are arranged in the electron configuration [Ne]3s 3p . Of these, four are valence electrons, occupying the 3s orbital and two of the 3p orbitals. Like the other members of its group, the lighter carbon and the heavier germanium, tin, and lead, it has the same number of valence electrons as valence orbitals: hence, it can complete its octet and . Learn how silicon isotopes are used to trace processes and cycles in Earth and the solar system. Explore the fractionation and applications of silicon isotopes in low .
Silicon is a nonmetallic chemical element in the carbon family, abundant in Earth's crust and cosmos. It forms compounds with oxygen as silica and silicates, and has various isotopes with different atomic masses.
Isotopes. Atoms of the same element with different numbers of neutrons. Key for isotopesFurther data for naturally occuring isotopes of silicon are listed above. This table gives information about some radiosotopes of silicon, their masses, their half-lives, their modes of decay, their nuclear spins, and .

Silicon has three stable isotopes; Si-28, Si-29 and Si-30. Of these three naturally occurring isotopes Si-28 is the most abundant as it is produced in stars as well as during nuclear .
Si: isotope data. Silicon isotopes are used in a variety of applications. Si-28 has been suggested to improve the thermal conductivity of semiconductors. Si-29 is used extensively in NMR spectroscopy. Si-30 .silicon isotopes The unique heavy silicon isotope signature of cherts has been transferred to Archaean granitoids during an oceanic subduction process, which was probably responsible for the formation of felsic .The Solution . With its gas centrifugation isotope separation process, using state-of-the-art centrifuges, Orano Stable Isotopes is able to enrich silicon over 99,9% in 28 Si, but also to propose isotopically modified content in the 3 natural isotopes (28, 29 and 30). Orano offers a reliable, proven and efficient solution for isotopic separation of Silicon.Periodic Table--Silicon. Silicon has nine isotopes, with mass numbers from 25-33. 28 Si (the most abundant isotope, at 92.23%), 29 Si (4.67%), and 30 Si (3.1%) are stable; 32 Si is a radioactive isotope produced by argon decay. Its half life, after much argument, has been determined to be approximately 276 years (DeMaster,1980), and it decays by beta . Silicon (Si) is an element present in all natural environments, including the biosphere. It has three stable isotopes of mass 28, 29, and 30, respectively. Its isotopic composition is expressed in the delta notation δ 30 Si ( 30 Si/ 28 Si) and δ 29 Si ( 29 Si/ 28 Si) in per mil units normalized to a standard reference (see “ Delta, IsotopicIba pa Silicon isotope fractionation during core formation is likely because enrichment of heavy isotopes is expected in the phase with the ‘stiffer’ bonds 10, in this case the Si–O bonds in . The mass-independent silicon isotope data are reported as mass bias-corrected deviations from the NBS-28 standard in parts per million, using the μ-notation as follows: μ 30 Si = [(30 Si/ 28 Si .Isotopes: Silicon has 14 isotopes whose half-lives are known, with mass numbers 22 to 36. Naturally occurring silicon is a mixture of its three stable isotopes and they are found in the percentages shown: 28 Si (92.2%), 29 Si (4.7%) and 30 Si (3.1%). References.Atomic Weights and Isotopic Compositions for Silicon Isotope Relative Atomic Mass Isotopic Composition Standard Atomic Weight Notes : 14 : Si : 28 : 27.976 926 534 65(44) 0.922 23(19) [28.084, 28.086] 29 : 28.976 494 664 90(52) 0.046 85(8) 30 .
Silicon (Si) is an element present in all natural environments, including the biosphere. It has three stable isotopes of mass 28, 29, and 30, respectively. Its isotopic composition is expressed in the delta notation δ 30 Si ( 30 Si/ 28 Si) and δ 29 Si ( 29 Si/ 28 Si) in per mil units normalized to a standard reference (see “ Delta, Isotopic
Name of the isotope: Silicon-28; Si-28 Symbol: 28 Si or 2814 Si Mass number A: 28 (= number of nucleons) Atomic number Z: 14 (= number of protons) Neutrons N: 14 Isotopic mass: 27.976926535 (3) u ( atomic weight of Silicon-28) Nuclide mass: 27.9692465 u (calculated nuclear mass without electrons) Mass excess: -21.49279 MeV Mass defect: .
Name of the isotope: Silicon-29; Si-29 Symbol: 29 Si or 2914 Si Mass number A: 29 (= number of nucleons) Atomic number Z: 14 (= number of protons) Neutrons N: 15 Isotopic mass: 28.976494665 (3) u ( atomic weight of Silicon-29) Nuclide mass: 28.9688146 u (calculated nuclear mass without electrons) Mass excess: -21.89508 MeV Mass defect: . The equilibrium silicon isotope fractionation during the mantle-core differentiation resulted in the observed heavy isotope composition of the bulk silicate Earth (BSE). The equilibrium . Silicon Isotope Analysis. Silicon isotope compositions were determined on the water samples from Crescent Stream (austral seasons 2014−2015, 2015−2016, and 2016−2017). Si was pre-concentrated using MAGIC brucite precipitation (Brzezinski et al., 2003) with Si recoveries of 85 to 100%.Silicon isotope biogeochemistry is the study of environmental processes using the relative abundance of Si isotopes. As the relative abundance of Si stable isotopes varies among different natural materials, [2] the differences in abundance can be used to trace the source of Si, and to study biological, geological, and chemical processes. [1] Silicon-30 is a stable isotope containing 16 neutrons. 3.092% of natural silicon is silicon-30. 32 Si Silicon-32 is a radioactive isotope containing 18 neutrons. Silicon-32 is formed as a daughter particle from the reaction between cosmic radiation and atmospheric argon. It decays further by β- decay into 32 P with a half-life of 15,319 years.Silicon carbide (SiC) is one of the hardest substances known and used in polishing. Also the crystalline form is used in semiconductors. Get the facts about element Silicon (Si) [14] from the periodic table. Find physical data, electron configuration, chemical properties, aggregation states, isotope data (including decay trees) as well as some .
silicon isotopes Iba paName of the isotope: Silicon-38; Si-38 Symbol: 38 Si or 3814 Si Mass number A: 38 (= number of nucleons) Atomic number Z: 14 (= number of protons) Neutrons N: 24 Isotopic mass: 37.99552 (11) u ( atomic weight of Silicon-38) Nuclide mass: 37.98784 u (calculated nuclear mass without electrons) Mass excess: -4.17309 MeV Mass defect: 0.321988432 .
silicon isotopes|Iba pa
PH0 · what is silicon
PH1 · silicon isotope abundance
PH2 · radioactive isotopes examples
PH3 · list of radioactive isotopes
PH4 · isotopes definition for kids
PH5 · important uses for isotopes
PH6 · how many electrons does silicon have
PH7 · geochemistry of silicon isotopes
PH8 · Iba pa